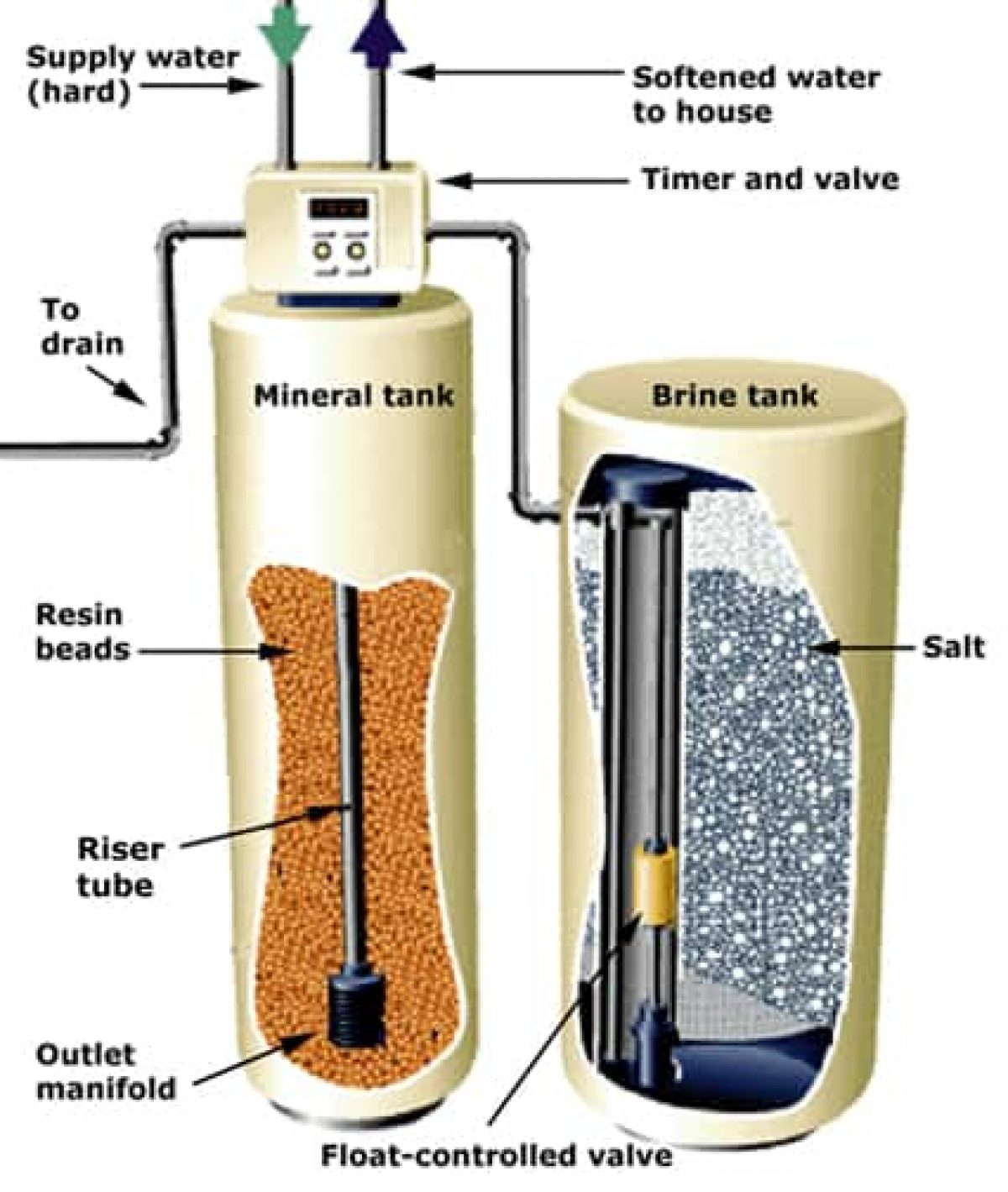
Water Softeners Chemistry . It works by passing the water through. Traditional water softeners use a process. An understanding of the chemistry of hard and soft water and the treatment process used to produce softer water can help you. Learn about about water softeners and how water. A water softener uses sodium to help replace calcium and magnesium ions. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. A water softener is a type of water treatment system that removes hardness minerals from a water supply. Chuck wight, a chemistry professor at the university of utah, provides the following explanation: A water softening experiment was conducted in replicate to observe the changes in parameters such as total hardness, calcium hardness,. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. On a small scale, chemicals.
from www.hometips.com
A water softening experiment was conducted in replicate to observe the changes in parameters such as total hardness, calcium hardness,. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. It works by passing the water through. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. An understanding of the chemistry of hard and soft water and the treatment process used to produce softer water can help you. A water softener uses sodium to help replace calcium and magnesium ions. Chuck wight, a chemistry professor at the university of utah, provides the following explanation: Traditional water softeners use a process. A water softener is a type of water treatment system that removes hardness minerals from a water supply. On a small scale, chemicals.
How a Water Softener Works
Water Softeners Chemistry Traditional water softeners use a process. A water softener uses sodium to help replace calcium and magnesium ions. On a small scale, chemicals. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Traditional water softeners use a process. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. Learn about about water softeners and how water. It works by passing the water through. An understanding of the chemistry of hard and soft water and the treatment process used to produce softer water can help you. A water softener is a type of water treatment system that removes hardness minerals from a water supply. A water softening experiment was conducted in replicate to observe the changes in parameters such as total hardness, calcium hardness,. Chuck wight, a chemistry professor at the university of utah, provides the following explanation:
From www.amazon.com
Aquasure Whole House Water Filtration Bundle w/Water Softener, 75 GPD Water Softeners Chemistry An understanding of the chemistry of hard and soft water and the treatment process used to produce softer water can help you. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. A water softener uses sodium to help replace calcium and magnesium ions. On a small scale, chemicals. A water. Water Softeners Chemistry.
From www.youtube.com
Learn How To Turn Hard Water Into Soft Water Environmental Chemistry Water Softeners Chemistry Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softening experiment was conducted in replicate to observe the changes in parameters such as total hardness, calcium hardness,. On a small scale, chemicals. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium.. Water Softeners Chemistry.
From sciencenotes.org
Hard Water vs Soft Water Know the Difference Water Softeners Chemistry Traditional water softeners use a process. An understanding of the chemistry of hard and soft water and the treatment process used to produce softer water can help you. It works by passing the water through. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a type of water treatment. Water Softeners Chemistry.
From netsolwater.com
How are Automatic Water Softeners useful Water Softeners Chemistry A water softener is a type of water treatment system that removes hardness minerals from a water supply. It works by passing the water through. Traditional water softeners use a process. A water softener uses sodium to help replace calcium and magnesium ions. A water softener is a device that removes hard minerals from the water supply, such as calcium. Water Softeners Chemistry.
From generationwatersystems.com
GenSoft EliteDual Commercial Grade Water Softener Generation Water Water Softeners Chemistry An understanding of the chemistry of hard and soft water and the treatment process used to produce softer water can help you. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. Chuck wight, a chemistry professor at the university of utah, provides the following explanation: A water softener uses sodium. Water Softeners Chemistry.
From www.slideserve.com
PPT Hardness of Water PowerPoint Presentation, free download ID2956417 Water Softeners Chemistry Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. It works by passing the water through. Chuck wight, a chemistry professor at the university of utah, provides the following explanation: On a small scale,. Water Softeners Chemistry.
From waterseer.org
6 Common Water Softener Problems (+ How to Fix Them) Water Softeners Chemistry Chuck wight, a chemistry professor at the university of utah, provides the following explanation: An understanding of the chemistry of hard and soft water and the treatment process used to produce softer water can help you. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. A water softening experiment was. Water Softeners Chemistry.
From purewaterblog.com
Citric Acid Water Softeners Everything You Need to Know Water Treatment Water Softeners Chemistry A water softener is a type of water treatment system that removes hardness minerals from a water supply. Traditional water softeners use a process. A water softening experiment was conducted in replicate to observe the changes in parameters such as total hardness, calcium hardness,. An understanding of the chemistry of hard and soft water and the treatment process used to. Water Softeners Chemistry.
From tinyhousegarage.com
How Does Water Softener Work Chemistry? Water Softeners Chemistry Chuck wight, a chemistry professor at the university of utah, provides the following explanation: Learn about about water softeners and how water. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. It works by passing the water through. An understanding of the chemistry of hard and soft water and the treatment process used. Water Softeners Chemistry.
From watersofteneruae.ae
Water Softener watersoftuate Water Softeners Chemistry Traditional water softeners use a process. A water softener uses sodium to help replace calcium and magnesium ions. Chuck wight, a chemistry professor at the university of utah, provides the following explanation: Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a type of water treatment system that removes. Water Softeners Chemistry.
From www.clearwatersystems.com
SmartSoft Water Softeners Water Softeners Chemistry A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. It works by passing the water through. Chuck wight, a chemistry professor at the university of utah, provides the following explanation: A water softening experiment was conducted in replicate to observe the changes in parameters such as total hardness, calcium hardness,.. Water Softeners Chemistry.
From waterfilterguru.com
How to Remove Salt from Softened Water (Top 3 Methods) Water Softeners Chemistry Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. A water softener uses sodium to help replace calcium and magnesium ions. An understanding of the chemistry of hard and soft water and the treatment. Water Softeners Chemistry.
From www.thespruce.com
Water Softeners How They Work Water Softeners Chemistry Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. On a small scale, chemicals. It works by passing the water through. Traditional water softeners use a process. A water softener uses sodium to help replace calcium and magnesium ions. Chuck wight, a chemistry professor at the university of utah, provides the following explanation:. Water Softeners Chemistry.
From netsolwater.com
What are the Guidelines for designing Water Softeners Netsol Water Water Softeners Chemistry Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a type of water treatment system that removes hardness minerals from a water supply. It works by passing the water through. Learn about about water softeners and how water. An understanding of the chemistry of hard and soft water and. Water Softeners Chemistry.
From patch.com
Water Softeners How they work Hartland, MI Patch Water Softeners Chemistry It works by passing the water through. Learn about about water softeners and how water. On a small scale, chemicals. Chuck wight, a chemistry professor at the university of utah, provides the following explanation: A water softening experiment was conducted in replicate to observe the changes in parameters such as total hardness, calcium hardness,. A water softener is a device. Water Softeners Chemistry.
From eyehomesolutions.com
Water Softener Systems How it Works and the Benefits Water Softeners Chemistry Learn about about water softeners and how water. Chuck wight, a chemistry professor at the university of utah, provides the following explanation: Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. Traditional water softeners. Water Softeners Chemistry.
From www.hometips.com
How a Water Softener Works Water Softeners Chemistry Chuck wight, a chemistry professor at the university of utah, provides the following explanation: Learn about about water softeners and how water. An understanding of the chemistry of hard and soft water and the treatment process used to produce softer water can help you. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange.. Water Softeners Chemistry.
From avidwatersystems.com
Water Softeners Avid Water Systems Water Softeners Chemistry Learn about about water softeners and how water. A water softener is a device that removes hard minerals from the water supply, such as calcium and magnesium. A water softener uses sodium to help replace calcium and magnesium ions. Water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Chuck wight, a chemistry professor. Water Softeners Chemistry.